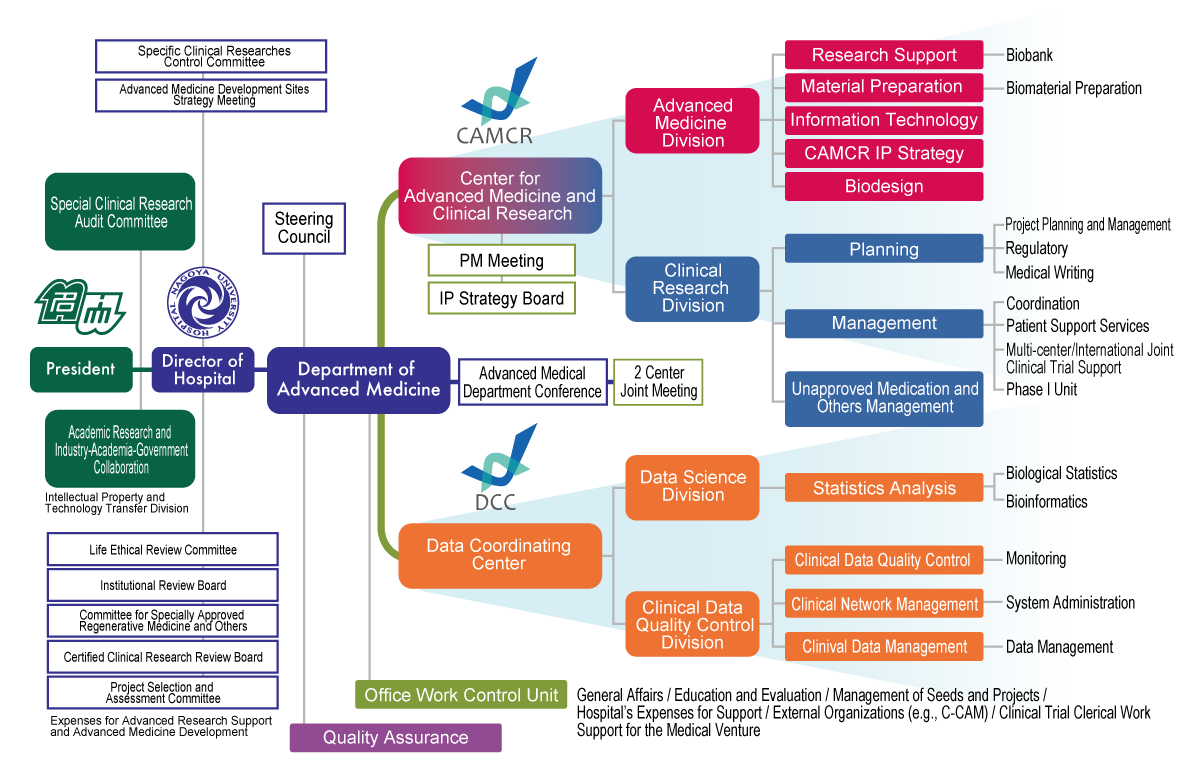
Quality Assurance Section
To assure that the clinical study was conducted and its data were prepared, recorded, and reported in compliance with regulations, guidelines, and others and in accordance with the approved protocol.
Office Work Control Unit
General Affairs, Education and Evaluation, Management of Seeds and Projects, Hospital’s Expenses for Support, External Organizations (e.g., C-CAM) , Clinical Trial Clerical Work, Support for the Medical Venture